Innovative Solution Partner for Medical Applications
TSRC develops a range of new VECTOR SEBS products that are suitable for medical applications. A key distinguishing feature of medical grade SEBS versus typical materials is plasticizers free. Moreover, VECTOR SEBS products also provide superior mechanical strength, high clarity and excellent polyolefin compatibility. TSRC VECTOR SEBS meets stringent quality standards and delivers outstanding performance in medical applications such as IV bags / film, tubing, stoppers, liners, syringe plunger tips and closures.
VECTOR SEBS
VECTOR SEBS (Styrene-Ethylene/Butylene-Styrene copolymer) is designed for medical applications as alternative to PVC due to its eco-friendly feature. VECTOR SEBS passed ISO 10993-5 cytotoxicity test, USP Class VI test, complied with medical GMP production, and is well suited for general modification of articles in medical applications.
- Alternative to Polyvinylchloride (PVC) without plasticizer
- Wide formulation latitude – processable both in Oil Free & Oil-Extended
- High Polypropylene (PP) compatibility with excellent clarity
- Thermoplastics – easy processing & recyclable
- IV Bags / Film
- Medical Tubing
- Medical Stoppers & Liners
- PP Closures / PP Ports
IV Bags / Film
VECTOR SEBS is suitable to apply in medical single and multi-layer film. The designed structure provides good compatibility with Polypropylene (PP) at various ratio to fulfill desired features such as printability, flexibility, and good sealing strength. In addition, the high flowability of VECTOR SEBS enables non-oil extended process available to meet medical requirements. The material also passes USP class VI test and ISO 10993-5 cytotoxicity test to prove the reliability of medical products.
Features
High clarity
Good impact resistance
Easy processing
Excellent compatibility with PP
Recommended Grades
VECTOR 8245D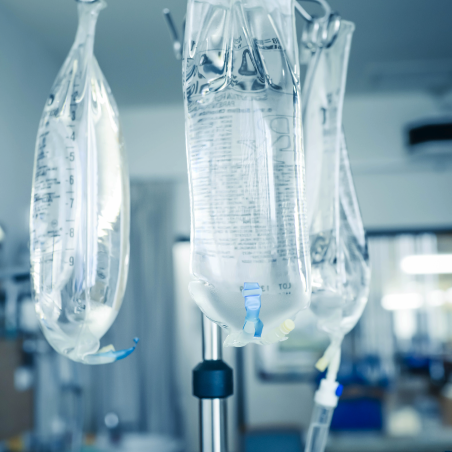
Medical Tubing
VECTOR SEBS is suitable for medical tubing applications due to its high flexibility, high transparency, and good softness as ideal alternative material to PVC without any plasticizer. By different ratio blending with Polypropylene (PP), the tubing hardness could be adjusted to meet different tubing requirements such IV tubing, suction tubing, dialysis tubing, etc. VECTOR SEBS passes USP class VI test and ISO 10993-5 cytotoxicity test to prove the reliability of medical products.
Features
High clarity
Good impact resistance
Good kink resistance
Easy processing
Excellent compatibility with PP
Recommended Grades
VECTOR 8245D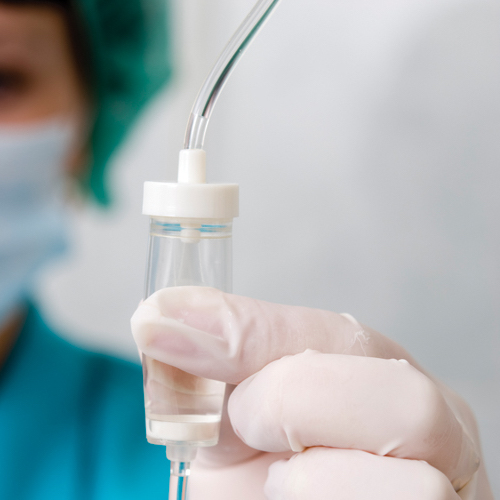
Medical Stoppers & Liners
VECTOR SEBS can be compounded with Polypropylene (PP) and paraffinic oil in common process for injection molding into various medical devices such as medical stopper and liner as ideal alternative material to vulcanized rubber like isoprene rubber (IR) and isobutylene isoprene rubber (IIR). VECTOR SEBS based compound in medical stopper and liner application has low fragment, good leak resistance after needle punctures. VECTOR SEBS passes USP class VI test and ISO10993-5 cytotoxicity test to prove the reliability of medical products.
Features
Leak resistance
Low fragmentation
Easy processing
Chemical inertness
Recommended Grades
VECTOR 8101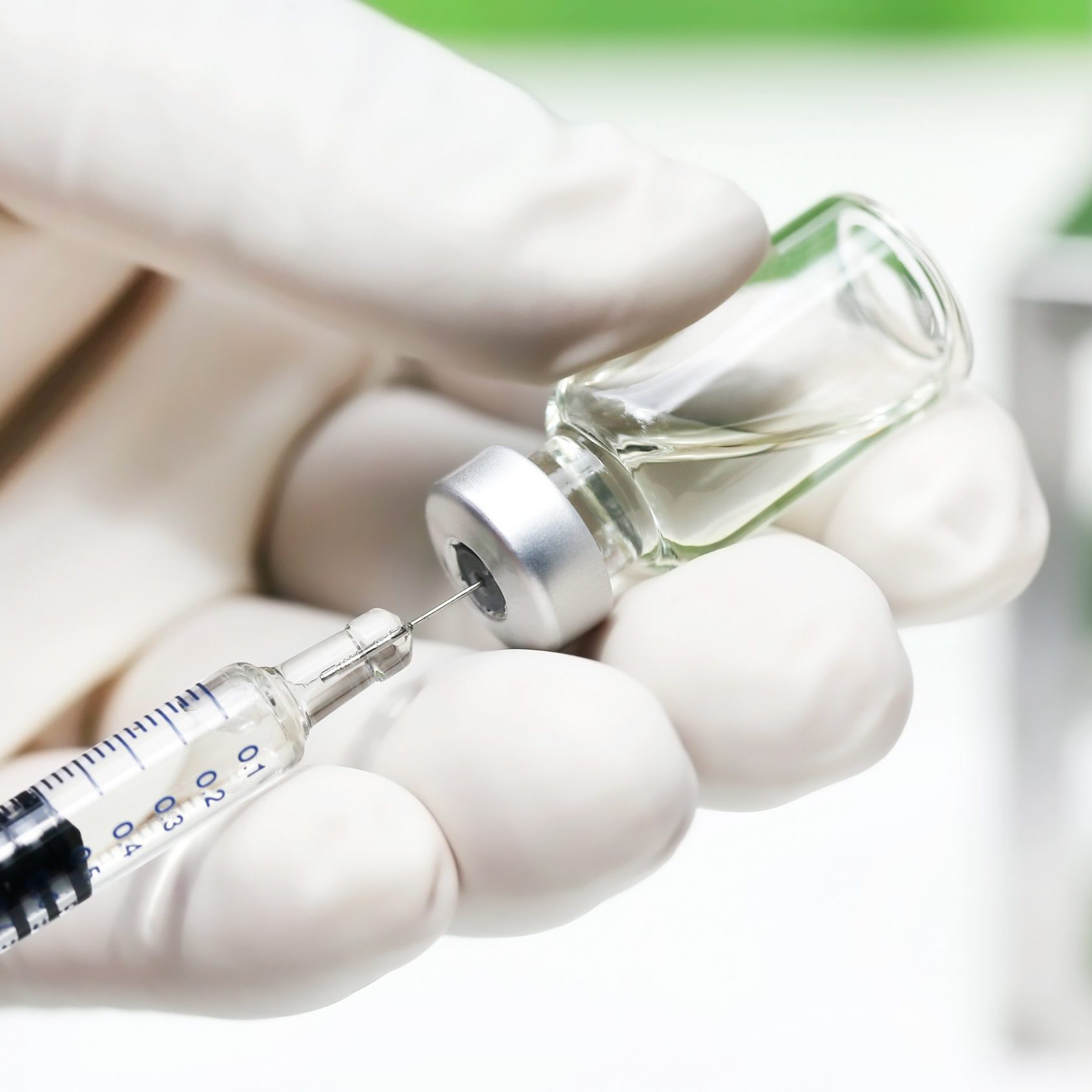
PP Closures / PP Ports
For non-PVC infusion system, Polypropylene (PP) closure and port are two of the key components which commonly use SEBS as modifier to enhance impact strength. VECTOR SEBS has good compatibility with PP and can be compounded in common process for injection molding. It also passes USP class VI test and ISO10993-5 cytotoxicity test to prove the reliability of medical products, suitable to apply in PP closure and port as plastic modifier.
Features
Leak resistance
Low fragmentation
Adjustable hardness
Recommended Grades
VECTOR 8101, 8103, 8104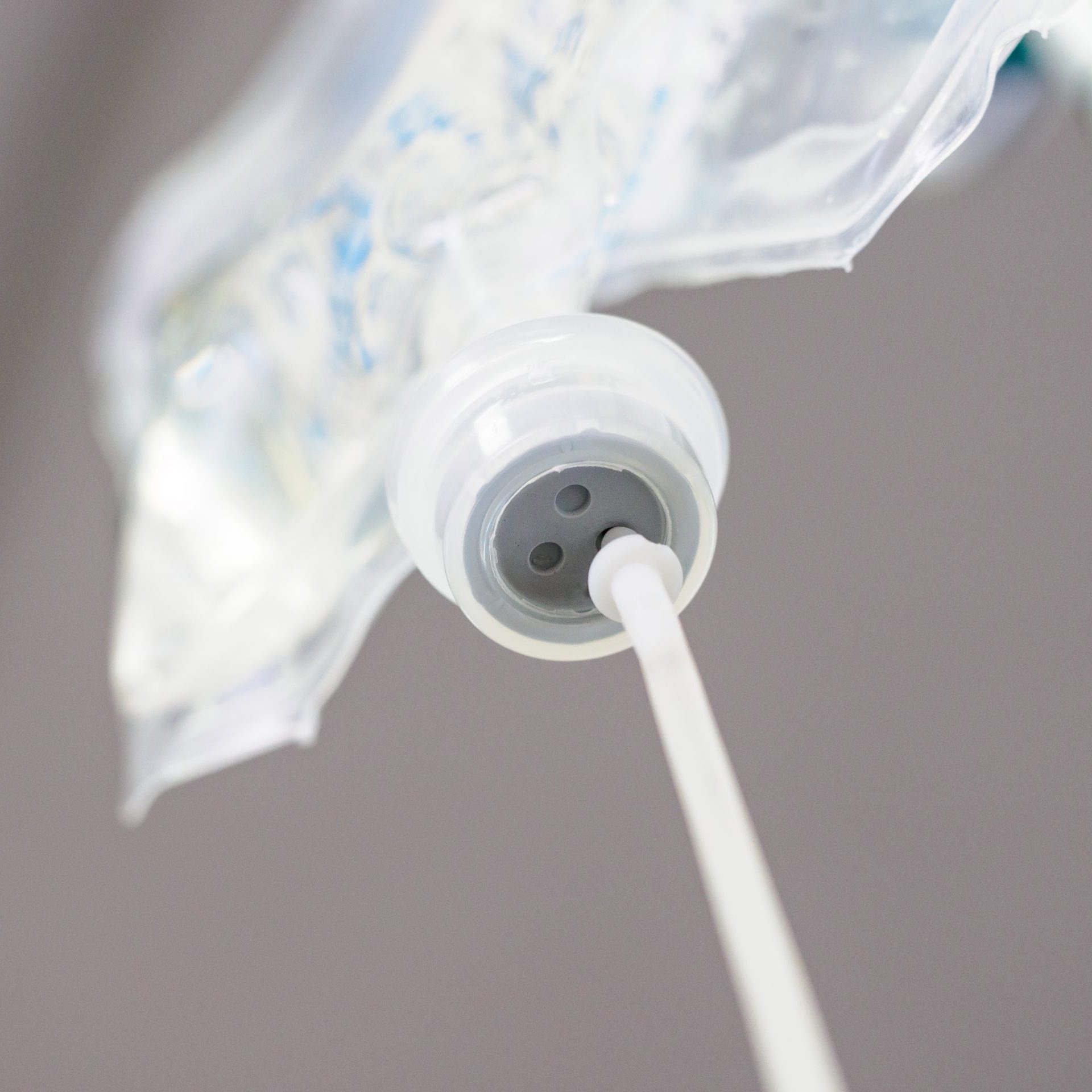
Medical SEBS in Polyolefin Modification
Effectively toughen PP while providing a wide range of properties
Features
- High vinyl SEBS with 13% styrene
- Excellent compatibility with PP
- High clarity performance
- Good impact resistance
- Medical GMP management
- Meet ISO 10993-5 Test
- Meet USP VI Test
- Easy processing without oil extended
Applications
- IV bag film / Continuous Ambulatory Peritoneal Dialysis (CAPD) bag film / Drainage bag film
- IV tubing / Dialysis tubing / Suction tubing
| Property | VECTOR 8245D |
|---|---|
| Hardness, Shore | 40 A |
| MFI (230℃ 2.16 kg) | 3.5 |
| Tb | 10 MPa |
| Eb | 1000 % |
| Transparency (3mm sheet) | 83.4 % |
| Rebound (45˚) | 68.9 % |
| Capillary@ 1000 s-1 | 151 Pa's |
| Viscosity@ 10000 s-1 | 27 Pa's |
Features
- A high molecular SEBS designed for medical compounds
- Low ash to meet Pharmacopeia
- Medical GMP management
- Meet ISO 10993-5 Test
- Meet USP Class VI Test
Applications
- PP closure / PP port
- Medical stopper / Liners
- Syringe plunger tip
Testing Formula
| Property | VECTOR 8101 |
|---|---|
| Hardness, Shore | 63A |
| Tb | 8.4 MPa |
| Eb | 822% |
| 300% Modulus | 2.4 MPa |
| Rebound (40˚) | 55.7% |
| Compression Set (100℃) | 49% |
TSRC Medical Declaration
A representative product, SEBS 8101, of VECTOR has been tested one time, and the results of the testing reports are summarized as follows:
Biological evaluation of medical devices- Part-5 Test for in vitro cytotoxicity
It met the criteria of biocompatibility guided by ISO 10993-5.
USP chapter <88> Biological reactivity tests, in vivo
It met the requirements of USP Class VI.
*This information is provided solely for customers to use in assessing whether VECTOR SEBS are appropriate for the customers’ intended end-uses. TSRC makes no warranties, express or implied, with respect to the merchantability, fitness, or suitability of vector sebs for any particular medical uses. The customer is solely responsible for ensuring that VECTOR SEBS is a suitable material for the customer’s intended application.